Abstract
Introduction: The management of venous thromboembolism (VTE) and the prevention of recurrent VTE consists of anticoagulation primarily with Vitamin K Antagonists (VKA). The main adverse effect of anticoagulation is bleeding. This study aimed to investigate the incidence and established and new predictors of significant bleeding occurring in primary care within 3 months after treatment initiation with VKA in patients with first VTE.
Methods: A cohort study was undertaken using the United Kingdom's primary care Clinical Practice Research Datalink with additional data from hospitalizations and causes of death. Patients with incident first VTE between January 2008 and March 2016 treated with VKA within 60 days after first VTE, were included in the cohort. Bleeding was defined as and either major bleeding or clinically relevant non-major bleeding requiring hospitalisation (CRNMB) in accordance with the International Society of Thrombosis and Haemostasis recommendations. Major bleeding and CRNMB events were reviewed, adjudicated, and validated by physicians experienced in care of VTE patients. Bleeding events that occurred in the hospital setting were excluded. Patients were followed for three months from VKA treatment initiation. Sub-hazard ratios (SHR) were estimated from Fine-Gray regression models with mortality as a competing risk and time-dependent covariates. Models included recognized predictors for major bleeding and CRNMB before the diagnosis of VTE and a list of potential predictors during VKA treatment.
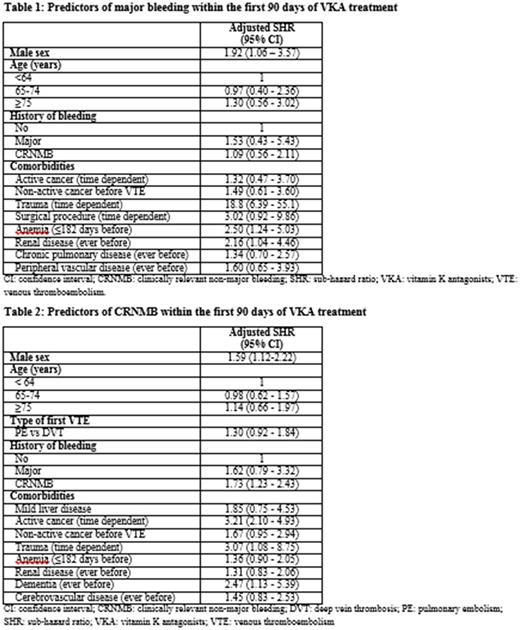
Results: Among 11,871 VKA-treated VTE patients there were 45 community-acquired acute major bleeding events and 146 CRNMB events requiring hospitalization in the first 3 months following commencement of VKA therapy. The three month cumulative incidence of community-acquired major bleeding and CRNMB was 0.4% and 1.3% respectively. Incidence rates for each type of bleeding peaked in the first two weeks after the start of VKA therapy. Independent predictors for major bleeding included male sex, adjusted SHR 1.92 (95%-confidence interval: 1.06-3.57), anaemia within 6 months before first VTE 2.50 (1.24-5.03), history of renal disease 2.16 (1.04-4.46), and trauma during VKA treatment 18.8 (6.39-55.1) (Table 1). Independent predictors for CRNMB included male sex 1.59 (1.12-2.22), active cancer 3.21 (2.10-4.93), trauma during VKA treatment 3.07 (1.08-8.75), history of dementia 2.47 (1.13-5.39) and history of CRNMB 1.73 (1.23-2.43) (Table 2).
Conclusions: Clinically relevant non-major bleeding requiring hospitalization occurred at least three times as often as major bleeding. Event rates are highest in the initial period following commencement of VKA therapy. Assessment for and awareness of predictors prior to and during VKA treatment are needed to prevent significant bleeding events. Caution is warranted early after commencing VKA, particularly in patients with prevalent risk factors.
Martinez: CSL Behrig: Research Funding; Bayer AG: Research Funding; Bristol-Myers Squibb: Research Funding; Merz Pharma: Research Funding. Cohen: Portola: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Katholing: Institute for Epidemiology, Statistics and Informatics GmbH: Employment. Wallenhorst: Institute for Epidemiology, Statistics and Informatics GmbH: Employment. Li: Bristol-Myers Squibb: Employment, Equity Ownership. Wygant: Bristol-Myers Squibb: Employment, Equity Ownership; University of Delaware School of Nursing Advisory Committee: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal